Mechanobiology in Spontaneous Healing of Anterior Cruciate Ligament
One of the main projects in our lab is to establish the spontaneous healing treatment strategy for the completely anterior cruciate ligament (ACL) injury. Traditionally, many researchers considered that a completely injured ACL does not heal spontaneously. In contrast, from the physical therapy perspective, we elucidated that controlling abnormal joint movement after ACL injury leads to spontaneous healing in the rodent model. However, the components and mechanical properties of the healed ACL are fragile compared to the intact ACL. Although these are common issues in the field of ligament and tendon healing, the mechanisms of the healing process and optimal rehabilitation led to normal tissues remain unclear.
Our research is a collaborative effort that involves experts from various fields, all working towards a common goal. To elucidate the remodeling process of a healed ACL, we focus on the mechanisms of ACL development and how mechanical stress promotes ACL remodeling. We investigate the healing mechanism of the ACL from multiple aspects using histology, mechanical testing, multiplex assays, and transgenic models. Our goal is to provide fundamental scientific insights into ACL conservative treatment and the mechanisms of ligament and tendon healing. By working together, we can make significant strides in our understanding of ACL injuries and their treatment, and we are excited to share our findings with the larger scientific community.

DAPI
Col1
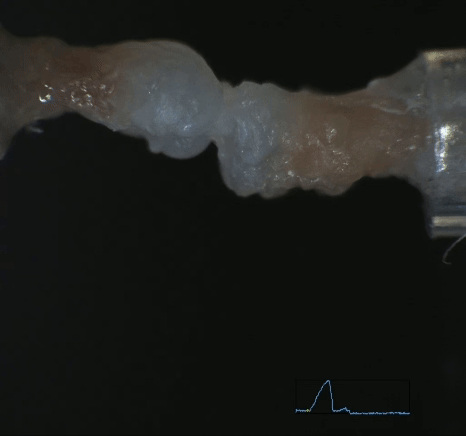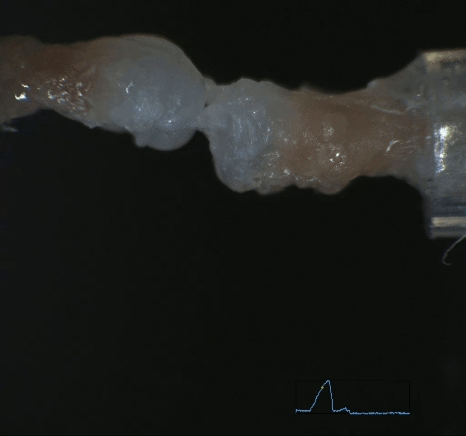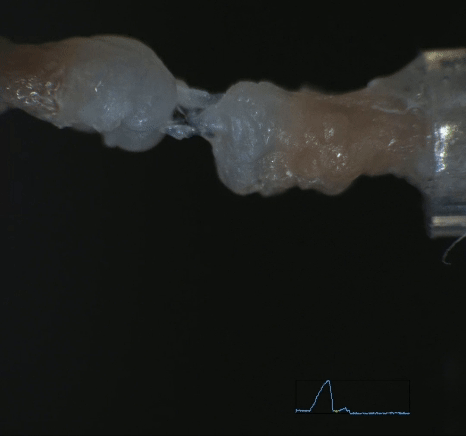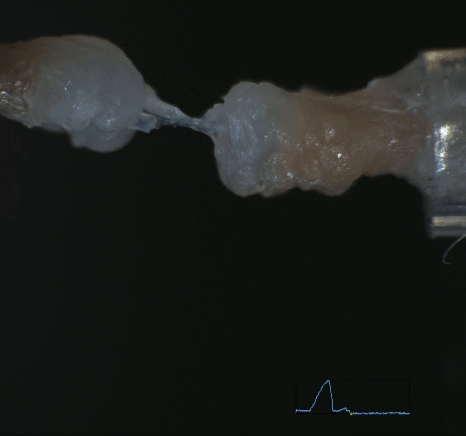
Mechanobiology in Tendon Development
This research project aims to understand the mechanobiological mechanisms that regulate tendon development. The musculoskeletal system provides structural stability and coordinates to enable movement. The tendon has the essential role of efficiently transmitting force generated from muscle contraction to bone to allow for ambulation so that they can resist high external forces. This force is required to maintain tendon growth and differentiation. This project focuses on identifying how mechanical force, such as muscle contraction and joint movement, promotes tendon development. Our group is using a murine surgical model and genetics models. Information gathered from the characterization of the mechanics and composition of assembling tissues will guide the design of more successful physical therapeutic strategies and provide success criteria for healthy tendon development and healing.


The Mechanisms of Tendon Healing and Functional Recovery of The Muscle-Tendon Complex After Achilles Tendon Rupture
This study focuses on developing effective rehabilitation after Achilles tendon rupture. The main problem after this injury is that the muscle-tendon complex dysfunction remains permanent. Tendons are originally avascular and hypocellular tissues with a low intrinsic healing capacity. Healed tendons are mechanically, structurally, and functionally inferior to normal tendons. Also, muscle dysfunction remains for several years. Therefore, establishing treatment strategies that comprehensively resolve these issues is needed.
Our proposed novel rodent model, which involves a unique combination of ankle immobilization/ triceps surae paralysis and additional interventions of passive stretching/ muscle strengthening exercise, is a departure from previous studies with unclear movement pattern definitions. These models aim to redefine the role of exercise in the muscle-tendon complex and provide a clear understanding of the effects of muscle contraction and passive ankle mobilization.
At this stage, we have shown that inhibiting muscle contraction is detrimental to tendon healing, while the presence or absence of passive joint motion does not contribute to tendon healing. Using these elaborate controlled models of movement patterns and the knowledge of mechanobiology, we are investigating the mechanisms and optimal rehabilitation protocols that affect the functional recovery of the muscle-tendon complex from multiple perspectives.
These studies provide fundamental knowledge for selecting appropriate management. They will contribute to establishing treatment strategies that can help patients fully return to their pre-injury activity levels.


Elucidating the Effect of Exercise Interventions to Promote Tendon Healing and Functional Recovery of Shoulder after Rotator Cuff Reconstruction
This project aims to elucidate the mechanobiological mechanisms inducing strength and functional recovery of tendons and enthesis and muscle function after rotator cuff tear. Rotator cuff tear is a common musculoskeletal disease, and the postoperative re-tear rate is still high. Our focus is on the role of mechanical stress through exercise in promoting tendon and enthesis healing. Mechanical stress has been reported to be essential for tendon and enthesis development and healing. However, appropriate exercise protocols such as intensity, frequency, start timing, and duration to promote tendon and enthesis healing after rotator cuff tear surgery are unknown. The healing process of the tendon is gradual so that the same exercise may be loaded differently at different times. The rotator cuff also shows unique muscle degeneration, such as atrophy, fatty degeneration, and fibrosis. So, the forces applied to the tendon and enthesis, which are responsible for transmitting muscle contraction forces to the bone, are thought to vary according to the condition of the muscles. Therefore, the mechanical properties of the tendon and the muscle condition need to be considered when examining the effects of exercise. We investigate the role of mechanical stress on tendon and enthesis healing using exercise devices, histology, biomechanical analysis, and shoulder function testing. We aim to provide essential scientific insights into rehabilitation in rotator cuff tears.

Controll

Injured
Exploring the Onset Mechanism of Knee Osteoarthritis in terms of Synovial Fluid
One of the main projects in our lab is to explore the onset mechanism of knee osteoarthritis (OA). Knee OA develops irreversibly as a whole joint disease with cartilage degeneration, osteophyte formation, synovitis, and subchondral bone changes. Cartilage is an avascular tissue and has poor self-healing capability. Thus, it’s essential to establish a prevention strategy for knee OA. Our lab focuses on abnormal mechanical stress in the knee joint as an OA onset factor, and we recently developed a novel murine model that can reproduce mechanical stress by non-invasively rupturing anterior cruciate ligaments. (Takahata, et al. Osteoarthritis and Cartilage. 2023)
This model causes mechanical stress as much as possible since, unlike conventional mechanical-induced animal models, there is no damage to other surrounding tissues. Using this model, we revealed that mechanical stress stimulated chondrocytes to produce Matrix metalloproteinase first, and then synovial cells reacted similarly. It was also clarified that synovitis was induced secondary to cartilage degeneration under mechanical stress.
As the next step, we try to reveal the molecular biological mechanism in terms of synovial fluid, which is a body fluid filled within a joint capsule. Synovial fluid plays a significant role in maintaining cartilage homeostasis by supplying nutrition, meaning that it can be used as a biomarker of early joint degeneration. We aim to clarify the OA development mechanism, find the onset time point earlier, and then establish the prevention protocol from a rehabilitation perspective.


Angiogenesis and Mechanobiology of Knee Osteoarthritis Pain
The primary goals of this groundbreaking research project are to understand the novel mechanisms of angiogenesis and the mechanobiology that controls the pain associated with knee osteoarthritis (KOA). Previously, the pain of KOA has been attributed to an inflammatory reaction in the joints. Therefore, drug injections have been used for treatment, but they have yet to result in complete pain relief.
In rehabilitation, it has been reported that exercise alleviates pain after KOA. However, there is a lack of evidence for a clear cause-and-effect relationship. We intend to study how exercise reduces pain by focusing on factors outside the joints. Based on the reports supporting the view that pain in part KOA is caused by abnormal neurogenesis of extra-articular blood vessels, we aim to clarify what causes abnormal angiogenesis in KOA using our novel KOA murine model.
We aim to provide fundamental scientific insights into the mechanisms by which KOA pain occurs.

Elucidation of the Mechanism of Knee Osteoarthritis Development Focusing on Sex Differences
This research project aims to elucidate the impact of sex differences on the development of knee osteoarthritis (KOA). Being female is a well-known risk factor for KOA. Among life events in women, pregnancy and childbirth are particularly notable, as they involve not only hormonal changes but also weight gain and shifts in body alignment. These effects of the perinatal period on women's bodies have been overlooked because of its being far too long from the onset of KOA. We hypothesized that such temporary physiological and biomechanical changes could have lasting effects on knee joint health. In this project, we are conducting animal experiments using mice to investigate how being female influences the onset and progression of KOA, with a focus on hormonal factors, metabolic function, and mechanical stress.

The Significance of Mechanical Stress in the Developmental Process of the Meniscus
The primary goal of this research project is to elucidate the effects of mechanical stress on the developmental process of the meniscus. The meniscus plays an important role in ensuring the load response of the knee joint and stability during joint motion, etc. Its distribution of blood vessels and cells is localized to the outer region, and this specific structure is formed during early postnatal development when posture and movement are altered. Mechanical stress is essential for the development of the musculoskeletal system. However, its details have not been clarified in the meniscus. This project focuses on explaining how mechanical stresses, such as compressive and shear stresses, applied to the meniscus, which is located between bones, affect the developmental process of the meniscus. Gait, histology, vascular structure supply, and cell cycle analysis are used to explore the details of the developmental process of the meniscus. This study aims to elucidate the mechanical properties and biological mechanisms of the meniscus and provide fundamental insights into its normal development and healing.


Tenocyte Mechanobiology
The goal of this research project is to elucidate the influence of the mechanical environment on tenocytes. Tendon healing proceeds through three phases: inflammation, proliferation, and remodeling. During these phases, the hardness of the extracellular matrix (ECM) varies, and the tenocyte responds to these varying substrate hardnesses. In addition, we hypothesize that mechanical memory also affects the activity of tenocytes during the healing phase. However, the association of the mechanobiology of tendons and tendon healing remains unclear. To elucidate the mechanobiology of tenocytes, we investigate the analysis of gene expression, protein, and exosomes. Our goal is to provide fundamental scientific insights and mechanisms into the tendon healing process.









